Challenge
Geisinger Health, like many large-scale healthcare institutions, used manual processes to store and replenish medical supplies. Because manual processes can be a source of human error, Geisinger made an investment in technology that uses the latest identification standards in medical devices to eliminate manual processes, beginning in areas that use lifesaving implants, such as heart catheterization labs.
Solution
Geisinger teamed with Owens & Minor to implement QSight®, a cloud-based inventory management system, to leverage the data in barcodes affixed to medical devices for the U.S. Food and Drug Administration (FDA) Unique Device Identification (UDI) Rule. Geisinger can easily capture and store a product’s Global Trade Item Number® (GTIN®) - the most widely used UDI standard - and other critical data with a simple scan.

Benefits
readily available. It allows clinicians to interface with both the electronic medical record (EMR) and an individual’s electronic health record (EHR) with detailed recordkeeping
for more accurate documentation.
to cost and patient outcomes.
Geisinger Health has been a leader in healthcare innovation, seeking solutions to modern healthcare challenges and adopting technologies in service to its patients, caregivers, students, and community. This commitment to innovation is one of the reasons Geisinger Health can boast more than a century of service to its Pennsylvania communities, and why research and consulting firm Gartner consistently ranks Geisinger in the Healthcare Supply Chain Top 25. What truly sets Geisinger apart is its exceptional due diligence in leveraging every possible capability from its supply chain. At Geisinger, any new technology implemented to meet a challenge is scrutinized for other features that can introduce efficiencies to other departments, other practices, other facilities. “Technology right out of the box might have more capability than you initially need, but you need to create a roadmap for enabling other features that will increase the performance of the business,” says Kevin Capatch, director of process engineering at Geisinger Health. A case in point is Geisinger’s comprehensive data and inventory management program that uses increasingly critical supply chain data to drive patient safety within its areas of care. Geisinger leverages the same codes required by the U.S. Food and Drug Administration (FDA) regulations for unique device identification (UDI) and automatic identification and data capture (AIDC) encoded on medical devices to identify products in inventory.
Pencil and Paper to Keyboard
“Over a decade ago, I watched as a well-intentioned cardiac catheterization technician managed his inventory storage area using paper and pencil, then keyed his shopping list into an enterprise resource planning (ERP) system template to reorder,” says Capatch. Manual processes like these were rife with potential for error and were occurring in every procedural area of the Geisinger system. Moreover, hospital technicians might be tempted to over-order or unintentionally pass over products close to expiration, costing the institution valuable resources – or worse yet – facing an out-of-stock on a critical device needed by a patient. And with no system to manage the dates, expirations were inevitable. “The combination of capturing information with a barcode scan and having a system that can use and act on that captured information has allowed Geisinger to turn the tide on managing expirations in our procedural areas.”

After this encounter, Geisinger opted to introduce technology that would eliminate manual processes governing inventory and eliminate the three “nevers” in healthcare supply chains: never run out of a critical product, never waste inventory due to product expiration, and never fruitlessly search for recalled items.
Geisinger teamed up with Owens & Minor, a global healthcare solutions company, to use its QSight® inventory management platform.
“QSight gives us product visibility from the moment a product comes into Geisinger, until it is either used with a patient, expired, disposed of, returned, or recalled,” Capatch says. “It brings inventory control, it brings functionality for recalls, it brings expiration management, it brings lot and serialization control – the inherent benefits we set out to acquire by instituting an inventory management program.”
A Standard of Supply Chain Care
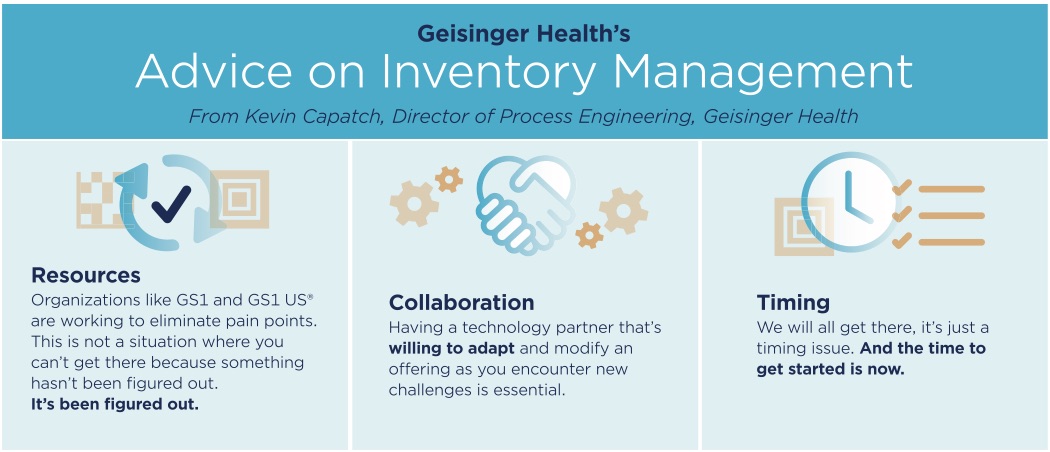
Data standards development had been underway for a few years when Geisinger and O&M began working together. In fact, data standards development continues to this day throughout health care and adjacent industries. As Capatch points out: “You must enforce [standards] in all disciplines: internally in your technology, in your business processes; externally with your vendor community and your solution providers.”
GS1 Standards – including Global Trade Item Numbers (GTINs) and Global Location Numbers (GLNs) – are the preferred and prevalent standards used by the healthcare community, pharmaceutical firms, medical device manufacturers and distributors, and are accredited by the FDA to support UDI implementation for all classes of medical devices. Eighty-seven percent of all UDI submissions utilize GS1 Standards, which work in conjunction with standards unique to health care1. But many entities in the healthcare ecosystem have yet to adopt GTINs and GLNs, which adds a level of complexity to any inventory management effort.
“We are proponents of GS1 Standards. If a vendor is on the fence, or deciding about upgrading business systems, we strongly encourage them to move to GS1 Standards. Theirs are more global. It’s a lot easier to interpret [GS1] application identifiers. This was an integral part in choosing our solution.” says Capatch.
Quality of Data Care
Data quality is imperative for optimal performance: it allows for seamless digital communications among systems, allows UDI data supplied by manufacturers to be used with confidence, and it powers inventory management.
“The importance of data quality is incalculable. Without clean data and a ‘single version of truth’ for all products coming into your system, you are hamstrung in what you can accomplish,” Capatch says. “That is why we benefitted from teaming up with a solution partner that made an investment in managing the item master data using standard identifiers where available, and we did not have to duplicate their efforts, just leverage them.”
“When working with a new client, the first thing we do is data cleansing,” says Vicky Lyle, vice president of Industry Associations at Owens & Minor. “We go into a [customer] department, scan each instance of every single product in that department to make sure the product exists in the global item master. In the instance where a product is not in the database, we utilize the GTIN to source the data from the GUDID [Global Unique Device Identification Database] or directly from the manufacturer’s site.”
Affiliates within the Geisinger system use QSight’s highly enriched item master database. A scan of GS1 barcodes on any given facility’s stockroom shelf will yield a product match of up to 95 percent. Once a product is added in the global item master, it is available for any customer that scans GS1 barcodes.
In the age of Big Data, hospitals are faced with collecting and interacting with massive amounts of data for thousands of products, most of which carry enriched attributes that go beyond manufacturers’ production information. A standards-based inventory management system, provides a secure repository and communications nucleus, bringing value to healthcare facilities like Geisinger needing to leverage the data in several vital ways, including patient safety.
In the age of Big Data, hospitals are faced with collecting and interacting with massive amounts of data for thousands of products, most of which carry enriched attributes that go beyond manufacturers’ production information. A standards-based inventory management system, provides a secure repository and communications nucleus, bringing value to healthcare facilities like Geisinger needing to leverage the data in several vital ways, including patient safety.
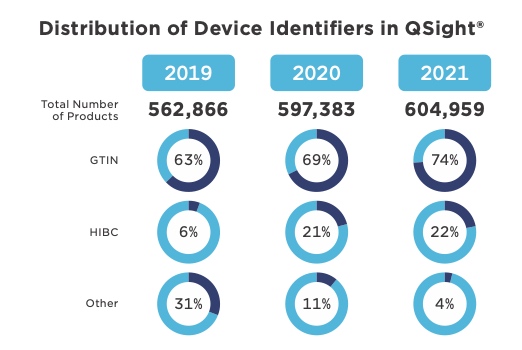
Currently, QSight has over 600,000 stock keeping units (SKUs) in its cloud-enabled database, which is available to all customers, with 74 percent of SKUs tied to a GS1 Global Trade Item Number® (GTIN®). QSight allows for flexibility so
that these alternate identifiers can be “tied” to the GS1 GTIN, when necessary, and supports the industry transition from proprietary identifiers to the standards-based UDI. QSight enhances its databases constantly when additional products are scanned by customers. And because it is cloud-based, the collective information is available to all customers as the scanning takes place, consistently recognizing a product via the GTIN encoded as the unique device identifier. That the constant evolution of QSight data is shared among all users benefits everyone.
“That’s where we’re starting to see some gains in using systems that are built on GS1 Standards,” Capatch says. “We can use standards to figure out what items we all have in common.”
The expansiveness of GS1 Standards provides a means of capturing specialized attributes that are crucial in tissue and bone implant procedures. A hip replacement appliance must include the side of the human body into which it is intended for use, for instance; tissue grafts may require information taken from a visual inspection or preparation of the biological material. The need for greater specificity to populate medical practice records as well as individual medical histories is satisfied using standards-based inventory management.
From Dock to Doc
The enterprise resource planning (ERP) system that purchases products is primarily quantity-based. ERP confirms receipt of a carton of 12 stents, for example, but does not note the 12 individual serial numbers or differing expiration dates. By making the connection via QSight between the quantity received and the enriched data connected to those products, visibility and inventory integrity is achieved.
For example, in the cath area, the receiving dock creates a delivery ticket using ERP data; QSight matches the ERP data to the requesting specialty inventory analysts in the cardiac catherization lab expecting delivery of the stents. Each stent is scanned individually – each with its own serial number along with its lot number and expiration date. From that moment on, Geisinger has visibility into each stent until it is used on a patient, is expired, or is returned to inventory because it was ordered in a case, but a larger stent was needed during the medical procedure.
A scan of the barcode will have captured everything about that stent and Geisinger’s inventory will reflect every step in the lifecycle of that product: ERP uses the incoming 5 shipment’s GTINs to match the source to the order placed. The cath lab personnel captures critical attributes – size, expiration data, lot/batch number – in addition to the unique identifier to link back to the manufacturer in the event of a recall. And nurses who scan the individual stent’s barcode in the procedure room allow the metadata to automatically populate the electronic medical record (EMR) and an individual’s electronic health record (EHR).
Although the industry continues to advance in standards adoption, exceptions still arise, so support of master data management by allowing for the recognition of products that are new to Geisinger is crucial. Product procurement can bypass ERP systems entirely, for instance. Stents from a new supplier might be ordered directly by the specialty department needing them (i.e., the cath lab). When the stents arrive at the point-of-care, the lab technicians scan the GS1 barcodes for supporting data for the devices, effectively introducing them into the master data system and placing them in the inventory management system.
Not only will the stents automatically be recognized at every subsequent juncture when scanned within the hospital, the data is automatically available within QSight where its cloud-based model of data distribution makes it available to all customers simultaneously, consistently linking the same unique identifier or GTIN encoded in the barcode to the product.
“When a barcode scan matches, almost everything else happens effortlessly,” Capatch says. “You don’t have people looking at descriptions, and other identifies on the packaging, you know the product is in your catalog, and can become trackable in inventory.”
The Proof is in the Inputting
Geisinger has successfully integrated inventory management fully into all non-OR procedural areas – including the catherization (cath labs), electrophysiology, and interventional radiology labs.
“The cath lab was first, but we didn’t have to do a lot of convincing after the first implementation: ‘That’s so much better than my paper-and-pencil grocery list inventory system!’ and ‘I don’t have to worry about expirations anymore because the system tells me you’re 90 days out on this item’ were common reactions from other departmental areas within Geisinger. Departments lined up. It was virtually self-marketing for the next installation,” Capatch says.
While the three initial practice areas are using QSight for 100 percent of their product tracking, inventory management, and data governance, Geisinger is now concentrating on bringing the operating rooms managing tissue and implants into full utilization.

“ORs are a little bit more challenging. You’re getting into a world where the identifier may have been on the packaging of the non-sterile item, and that identifier gets removed when it’s put into the sterilizing case. So, we are figuring out how we’re going to capture data on things like non-sterile implants,” Capatch says.
Capatch adds, “Without end-to-end inventory management, a nurse in the OR might have to employ multiple tools to scan multiple implants, which is not just bothersome, it’s potentially dangerous. And without standards, none of the automatic identification of products is possible.”
The Wide View
Enabled by standards-based master data and the advanced data analytics available in QSight, Geisinger can drill-up to gain supply chain insights or drill-down to the case level to get the information the healthcare system needs, aggregated in the way it chooses.
Geisinger’s extensive inventory management efforts provide the underpinning for a system-wide recall process. The system’s assimilation of GS1 Standards facilitates visibility – not just into individual products in inventory – but into the network’s entire operations by supplying reliable parallel comparisons – the kind of analytics needed to improve performance, which in the case of health care, can have profound consequences such as patient safety, expense management, and reimbursements. Standards open the door even wider: multiple hospitals can team-up to conduct life-saving research into health outcomes when apples-to-apples analyses are achieved through standards. These forward-thinking initiatives on Geisinger’s roadmap will all be enabled by leveraging data made available by scanning the barcodes on medical devices.

About the Organizations
|
Geisinger Health is a healthcare system located in central Pennsylvania serving one million people. Founded more than 100 years ago by Abigail Geisinger, the system now includes 10 hospital campuses, a health plan with more than half a million members, a Research Institute and the Geisinger Commonwealth School of Medicine. With nearly 24,000 employees and more than 1,600 physicians on staff, Geisinger boosts its hometown economies in Pennsylvania by billions of dollars annually. www.geisinger.org |
|
Owens & Minor, Inc. (NYSE: OMI) is a Fortune 500 global healthcare solutions company integrating product manufacturing and delivery, home health supply, and perioperative services to support care through the hospital and into the home. Owens & Minor drives visibility, control and efficiency for patients, providers and healthcare professionals across the supply chain with proprietary technology and solutions, an extensive product portfolio, an Americas-based manufacturing footprint for personal protective equipment (PPE) and surgical products, as well as a robust portfolio of products and services for patients managing chronic and acute conditions in the home setting. Operating continuously since 1882 from its headquarters in Richmond, Va., Owens & Minor is a 140-year-old company powered by more than 20,000 global teammates. www.owens-minor.com |

About GS1 Healthcare US
|
GS1 Healthcare US® is an industry group that focuses on driving the adoption and implementation of GS1 Standards in the healthcare industry in the United States to improve patient safety and supply chain efficiency. GS1 Healthcare US brings together members from all segments of the healthcare industry to address the supply chain issues that most impact healthcare in the United States. Facilitated by GS1 US, GS1 Healthcare US is one of over 30 local GS1 Healthcare user groups around the world that supports the adoption and implementation of global standards developed by GS1. www.gs1us.org/healthcare |
About GS1 US
|
GS1 US®, a member of GS1® global, is a not-for-profit information standards organization that facilitates industry collaboration to help improve supply chain visibility and efficiency through the use of GS1 Standards, the most widely used supply chain standards system in the world. Nearly 300,000 businesses in 25 industries rely on GS1 US for trading-partner collaboration that optimizes their supply chains, drives cost performance and revenue growth while also enabling regulatory compliance. They achieve these benefits through solutions based on GS1 global unique numbering and identification systems, barcodes, Electronic Product Code-based RFID, data synchronization, and electronic information exchange. GS1 US also manages the United Nations Standard Products and Services Code® (UNSPSC®). www.gs1us.org |